Dezember 12, 2024
Research
How the Latest Sensors Analyse Body Fluids
A new generation of wearable sensors will fundamentally change medicine. Researchers at ETH Zurich and international experts have now published an overview showing what is possible with such sensors and what questions their developers should consider to ensure their successful future use.
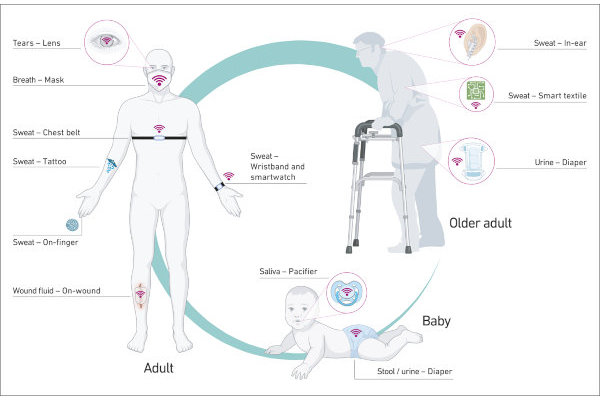
Various sensors might be helpful at different ages. (Image: Brasier et al. / Nature, 2024)
Using a smartwatch to measure pulse, and a smartphone app to monitor blood pressure: wearable sensors already track some of the body’s vital functions fairly reliably, and some of these devices can already be used in clinical diagnostics. However, diagnoses based on biochemical data still require samples of body fluids such as blood and urine, which have to be sent to the lab for analysis. Collecting these can be painful and complicated as well as time-consuming and often costly.
But the next generation of wearable sensors is set to provide biochemical analyses as well. In the future, such sensors will gather valuable insights into their wearer’s state of health by analysing body fluids such as sweat, breath, saliva, tears and urine. Although many of these advances are not yet ready for the market, they are certainly feasible. This is what led Dr Noé Brasier, Early-Career Fellow at the Collegium Helveticum, and ETH Professor Jörg Goldhahn to join forces with leading researchers in the field of wearable sensors and conduct a comprehensive review. Their overview was recently published in the journal Nature.
From infants to senior citizens
The advantages of wearable sensors are obvious: they allow continuous monitoring of health variables without patients having to visit a doctor’s surgery or pharmacy. “For elederly people who suffer from heat stress, it would make life a lot easier if a wearable device could remind them in good time to drink enough, or if a sensor could sound an alarm when their electrolytes reach a critical level,” says Brasier, himself a physician and the paper’s lead author.
Moreover, such sensors are either non- or only minimally invasive. Brasier provides an example: “Attempts to take blood from babies and infants, not to mention to insert a catheter, aren’t always successful. This can lead to significant delays, and is often distressing for the young patients and their parents. It would be much easier and more convenient to have a sensor on the baby’s skin or in their nappy perform the laboratory and/or urine analysis.” Equally, face masks that were additionally capable of detecting viruses such as SARS-CoV-2 without the need for an unpleasant nasal swab would have been welcome during the last pandemic.
A lot is possible – but does it make sense?
The researchers’ creativity is impressive, as is the sheer variety of conceivable devices – ranging from a dummy that measures whether infants are dehydrated to tattoos that indicate blood sugar levels and contact lenses that provide data from the wearer’s tears. “When we discussed the possibilities with engineers, physicians and colleagues from other disciplines a year ago, we realised that we needed to think about what kinds of sensor make sense and what points ought to be given particular weight when developing such devices,” says Goldhahn, the paper’s senior author.
The key consideration is self-evident: the wearables must be something that patients want to wear. “That’s why we recommend always developing the sensors together with the people who will need them later,” Brasier says. But the medical benefits of such devices also need to be critically assessed. Not everything that can be measured offers a clinical benefit. “It’s not about measuring any old variable. It’s a question of what that reading means in the relevant context and what the clinical consequences are,” he says.
For example, CRP is a marker for inflammation in the body and is measured in milligrams per litre. In healthy adults, the CRP level is normally physiologically below 5 mg/l. “If a patient has a blood-CRP level of 150 mg/l, this only tells us so much. What’s decisive for a clinical assessment is whether the value on the previous day was normal, or whether it was 300 mg/l. Then we can say whether the person’s health has deteriorated or improved.”
Display the readings well
Then there are the technical hurdles: How long can a sensor keep measuring? How can it be stored and cleaned? How much electricity does it consume, and from what source? And most importantly, how good and reliable is the data it provides? “Careful validation of the measurement data will be key to whether a given device becomes established or not,” Goldhahn says, “because nobody is going to rely on uncertain readings.”
In a further step, the signals from the wearables must be processed, interpreted and displayed in a way that makes sense to users – be they the patients themselves or healthcare professionals. In the future, that will increasingly be a job for artificial intelligence, which in turn will further accelerate the development of wearables.
Fascinated by sweat
It was sweat that led the lead author Brasier to become acquainted with wearables. While many people turn up their noses at the thought of this body fluid, Brasier can’t speak of it highly enough: “Different situations will always cause us to sweat differently and on different parts of the body. But that’s not the only reason why our sweat contains an incredible amount of information.” Using this information is a simple and straightforward way to draw conclusions about someone’s state of health. “The surface of the skin is my clear favourite, but the choice of sensor naturally depends on the medical application. In the case of pneumonia, for instance, it’s probably better to analyse the patient’s breath,” Brasier says. However, having prepared the new overview, he is well aware that there is a lot of research and development work still left to do – not least when it comes to clinical concepts. Only then will the new wearables ultimately be granted official approval and provide a benefit for everyone involved, especially patients.
Reference: Brasier et al: Applied body-fluid analysis by wearable devices, Nature, 04 December 2024, doi: external page10.1038/s41586-024-08249-4
Author: Franziska Schmid, Corporate Communications
Source: ETH News
