Februar 27, 2018
Exclusive News
3D Printing is Breaking Through the Hype Cycle
You couldn’t ask for a more perfect fit with the “hype cycle,” a concept developed by information technology firm Gartner, than the trajectory of additive manufacturing/3D printing through the years. The five hype cycles—technology trigger, peak of inflated expectations, trough of disillusionment, slope of enlightenment and plateau of productivity—aptly describe the rise and fall and, if the stars align, rise of new technologies.
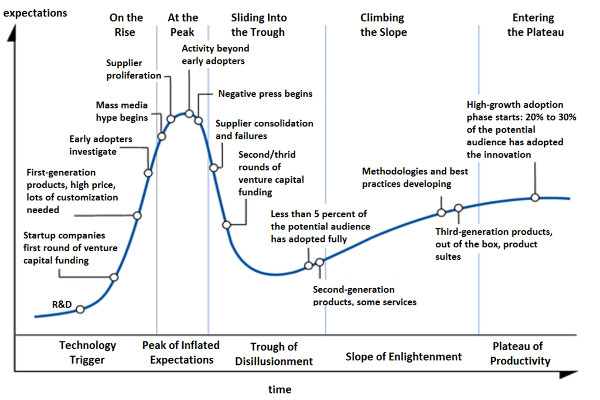
In the most recent classification of Gartner’s hype cycle, 3D printing of medical devices and bioprinting human tissue are classified as “sliding into the trough,” while dental applications are “climbing the slope.” Hearing aids are headed for the end game in the “plateau of productivity”: More than 90% of plastic shells for in-the-ear hearing aids are 3D printed, according to industry bible, the 2017 Wohlers Report. While the trough of disillusionment may not sound like a place you want to linger, it can be just part of the journey for a technology or application that was overhyped early on and must now prove its value through tangible results to climb the slope of enlightenment. From my perspective, that is where medtech 3D printing sits today, and here are a few reasons why.
3D printing enters residency in hospitals
3D-printing pioneers Stratasys and Materialise both struck deals with medical technology companies at the annual meeting of the Radiological Society of North America in November that resulted in embedding the technology more completely within the clinical workflow. Phillips’ advanced visualization platform IntelliSpace Portal 10 now includes a seamless interface with Stratasys’ technology, enabling radiologists to rapidly design and fabricate anatomical structures on demand. For its part, Materialise has partnered with the Siemens Healthineers platform to allow radiologists to print anatomical models using Materialise Mimics inPrint software. “This will facilitate the integration of 3D printing within clinical environments, contributing to higher quality, cost-efficient care for patients and hospitals,” said Materialise.
Stratasys also introduced BioMimics at the annual meeting, which provides exceptionally realistic 3D-printed anatomical models that are suited for use by surgeons preparing for a procedure as well as manufacturers testing device designs. BioMimics effectively mirrors intricacies of both soft tissue and hard bones via multi-material 3D printing, said Stratasys, eliminating restrictions associated with research on animal, mannequin, or cadaver models.
The crucial role that 3D-printed surgical models can play in achieving successful outcomes was illustrated recently when surgeons separated conjoined twins at the Bambino Gesù pediatric hospital in Rome. Preparations for the procedure took 11 months, and included 3D-printed models of the complex organ shape and vascular network of the young children, who shared an abdominal cavity, liver, rib cage, and sternum, reported 3ders.org. The models helped ensure that the 10-hour procedure achieved a successful outcome.
Taking the technology a step further, researchers led by the University of Minnesota in the United States have 3D printed lifelike models of patients’ prostates with integrated soft sensors. Silicone-based inks were used that can be precisely “tuned” to match the mechanical properties of each patient’s prostate tissue. Soft 3D-printed sensors were integrated in the organ models, allowing researchers to observe the reaction of the model prostates during compression tests and the application of various surgical tools. The technology could one day be used to produce bionic organs for transplants, said the researchers.
While we are still a long way from 3D-printing transplantable organs—an oft-repeated prediction during the early hype phase of medical 3D printing—progress continues, and the technology increasingly is being integrated into the clinical workflow. Look for healthcare establishments to increasingly adopt 3D printing technology within its infrastructure in 2018 and beyond. Considering the rapid pace of technological change, we can expect to see many more 3D-printed medical miracles coming down the pike through this decade.
Material matters
Silicon Valley-based Carbon made news this year when it introduced Silicone (SIL 30), a soft, tear-resistant, biocompatible resin that, the company said, opened up additive manufacturing applications for a range of medical and consumer products. The material is one of seven available from the company that have been certified biocompatible by medical research organization NAMSA.
SIL 30 earned its medtech bona fides when it was used to develop a pediatric stent that can be replaced as the child grows. Surgeons and researchers tried several other materials and 3D printing methods for this procedure but they failed because of the dynamic action of the airways, said Dr. Robroy MacIver, Congenital Heart Surgeon at Children's Minnesota.
Carbon’s Digital Light Synthesis technology and SpeedCell system represent a novel method for using 3D printing for production at scale and mass customization.
The year has seen a flurry of activity by chemicals companies striding into the 3D printing space, including BASF and Clariant establishing businesses dedicated to 3D printing. A focus of their activity is to develop compatible materials, the dearth of which continues to hamper the progress of 3D printing. Conventional plastics processors have thousands of polymers from which to choose, while only a handful exist for 3D printing. Medical-grade 3D printing plastics are even more sparse.
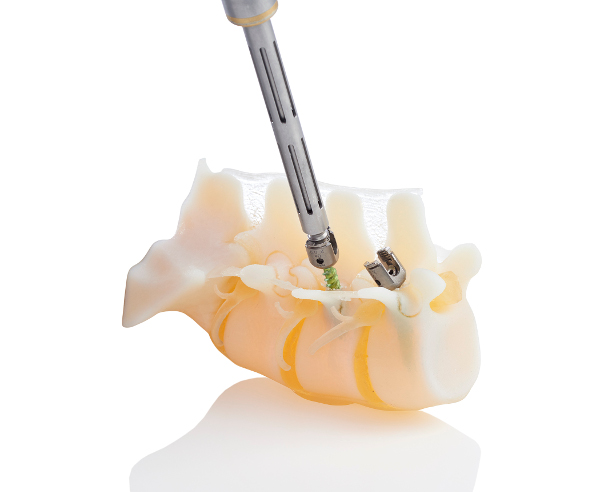
Custom-printed metal parts—mostly in stainless steel and titanium but research is ongoing into other metal powders—can be produced to precisely fit patient-specific anatomies and in record time. In Australia, neurosurgeon Ralph Mobbs used a 3D-printed spinal implant to bring relief to a patient suffering from severe pain in his back and leg because of a bulging disc and congenital defect in the lower spine. Applying design automation software from Australian company 3DMorphic to the patient’s CT scan, it reportedly took 20 seconds to produce a perfect-fit titanium implant that otherwise would have taken several hours.
German company Emerging Implant Technologies GmbH (EIT), which is specialized in titanium-based spinal implants, scored a major win this month when it secured a contract with the largest group purchasing organization in the United States. The Hospital Corporation of America (HCA), which operates almost 200 hospitals and more than 100 surgical centers in the United States and United Kingdom, has inked a deal with EIT to determine the economic impact of 3D printing on the medical sector. In July of this year, EIT’s 3D-printed Cellular Titanium spinal support implants received 510(k) clearance from FDA.
FDA steps up with guidance for makers of 3D-printed medical devices
On Dec. 4, FDA issued guidance for manufacturers of 3D-printed medical devices. While some pundits exclaimed, “it’s about time,” FDA pointed out that it is the “first regulatory agency in the world to provide a comprehensive technical framework to advise manufacturers creating medical products on 3D printers.” Recognizing that 3D printing of medical devices, medications and human tissues is quickly becoming a reality, FDA felt the time had come to provide a more comprehensive regulatory pathway in anticipation of “a significant wave of new technologies that are nearly certain to transform medical practice.”
The new Medical Device Regulation, on the other hand, is largely missing from this conversation. Under the regulation, 3D printed devices fall under the definition of custom devices. However, the regulation states that “mass-produced devices which need to be adapted to meet the specific requirements of the medical practitioner or other professional user shall not be considered to be custom made devices.” Consequently, as Erik Vollebregt, a legal professional specializing in the life sciences and co-founder of Axon Lawyers in Amsterdam, noted on LinkedIn, manufacturers of mass-produced 3D-printed parts will need to compile full technical documentation and quality management system data that are proportionate to the risk class of the device. The regulation considerably increases the requirements compared with the Medical Device Directive that it replaces, argues Vollebregt. Considering that companies such as HP and Carbon are knocking at the gates of production-scale manufacturing (although not yet for the medical space), the regulation appears less forward thinking than FDA.
While there are still hurdles to overcome, especially in terms of materials and regulatory pathways to market, 3D printing technology will continue to shake up conventional medical manufacturing and clinical practices in 2018 and beyond, and it will be well rewarded in terms of market growth. According to Allied Market Research, the global 3D printing healthcare market is expected to record a 26% compound annual growth rate through 2020 to reach a value of $2.3 billion. Trough of disillusionment? It’s looking pretty positive to me.
© MTME - MedTech Media Europe || Joseph Heeg
